We all own a smartphone, it has now become almost an extension of our body. Impossible not to have it: it avoids the queues in the offices, allows us to communicate with the institutions, helps us to see our distant loved ones, but … to some extent we have become dependent on it. Having it reassures us, comforts us, amuses us, makes us communicate. Information is at hand, communication is instant; we cannot do without it, we always carry it with us.
It is essential to be connected.
Given the great advantages it offers us, the improper use of an Internet-connected smartphone can hide problems and threats.
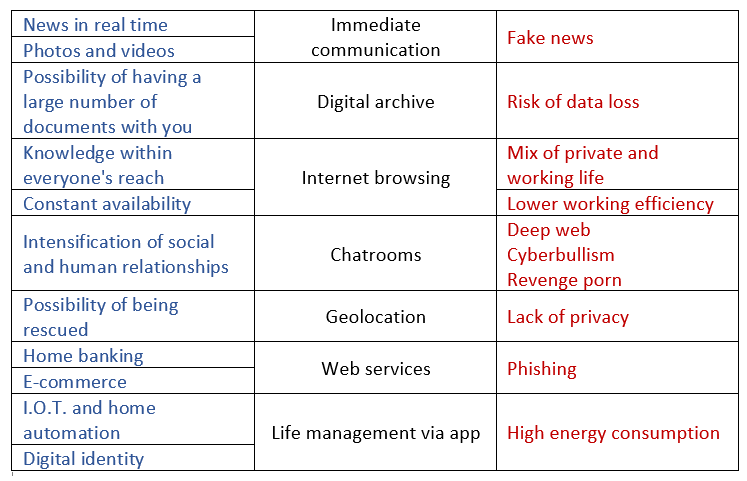
At this point, let’s ask ourselves what are the real costs associated with its use, its production and its disposal in terms of sustainability in all its conjugations.
Energy costs
“Being connected” has a big energy cost; the large servers that power the Internet consume 7% of global electricity which comes mainly from fossil sources: Facebook consumed 532 million kWh in one year; sending a 1 MB email produces 19 g of CO2.
5.9 billion people in the world have access to mobile phones and the trend is growing: in 2021, 1.39 billion smartphones were sold worldwide, compared to 1.29 billion units in 2020, with growth overall by + 4%.
In Italy 97.3% of those with an Internet connection own a smartphone. According to the Global Digital Report of February 2022 published by the We Are Social Agency, in Italy there are 78 million and 220 thousand cell phones, which compared to a population of 58,983,000 people residing in Italy as of January 1, 2022 (Istat data) give1,3 mobile phones each.

Costs in raw materials
Not everyone knows what materials a smartphone is made of and that each device contains a real treasure. E-waste Lab studies by Remedia, in collaboration with the Politecnico di Milano, have reckoned that a Smarthphone contains:
- 9 g of Copper
- 11 g of Iron
- 250 mg of Silver
- 24 mg of Gold
- 9 mg of Palladium
- 65 g of plastic
- 1 g of rare earths (Praseodymium, Neodymium, Cerium, Lanthanum, Samarium, Terbium, Dysprosium)
- tiny amounts of Cadmium, Cobalt, Ruthenium
And then there is the lithium-ion battery, which contains:
- 3.5 g of Cobalt
- 1 g of rare earths (Neodymium, Europium, Cerium and Terbium)
The conductors and micro capacitors instead contain Tantalum, obtained from Coltan.
Rare earths (REEs) are superconducting metals indispensable in all consumer, industrial and military technologies that will be indispensable in the next green energy conversion.
Why rare earths? In nature they are not found in purity, but linked to about 200 different types of minerals (halides, carbonates, oxides, phosphates and silicates) in quantities between 0.5 and 60 parts per million. The extraction is scarce and there are few mining sites.
The extractive environmental impact is important: separation requires a lot of water, energy and treatments with chemicals that also produce radioactive waste (Thorium and Uranium).
Great geopolitical problems arise from this: China owns most of the mining sites and extraction plants.


Coltan is indispensable for all electronic devices because it optimizes energy consumption in the new generation chips. Coltan is a complex mixture of columbite [(Fe, Mn) Nb2O6] and tantalite [(Fe, Mn) Ta2O6], two minerals of the oxide class that are very rarely found pure in nature. Hence the name Col + tan.
It is extremely rare in nature and therefore extremely valuable; it is a strategic resource for the development of new technologies.
80% of the world reserves of Coltan are found in Africa and 80% of these are in Congo.
The Congolese Coltan with a high concentration of tantalite is the most valuable on the planet and is therefore extremely important from a geopolitical point of view.
Human costs

Coltan is extracted with bare hands mainly by “slave” children, in open-cast mines controlled by armed militias.
Many of them die of fatigue and various diseases caused precisely by contact with Coltan. A report by Doctors Without Borders reports the following pathologies:
- Heart,blood vessels, brain and skin diseases;
- reduced production of blood cells and damage to the digestive system;
- increased risk of cancer;
- genetic defects in the offspring;
- diseases of the lymphatic system.


Environmental costs
In 2021, 57.4 million tons of electronic waste were produced worldwide. A crazy quantity that ends up in landfills every year, rarely in the recycling centers set up for the correct disposal of WEEE waste (Waste from Electrical and Electronic Equipment). In fact, of all electronic waste, only 17.4% is adequately treated and recycled.
If properly disposed of, more than 96% of its materials can be recovered from a telephone.
The recycling of one million mobile phones produces 24 kg of solid gold, 16 kg of copper, 350 of silver and 14 of palladium.
Only rare earths have a recycling rate of 1%, due to the lack of adequate facilities for collection and recovery.
Italy disposes of approximately 1.05 mobile phones per capita every year. With a percentage of 32.1%, over 365 thousand tons of waste from electrical and electronic equipment (WEEE) are sent for proper disposal. Fortunately, the trend is on the rise: in 2020, for example, there was an increase of 6.35% compared to 2019.
Furthermore, adequate recycling of a mobile phone avoids the emission of 0.211 kg of CO2 and leads to an energy saving of 1 kWh.
Each ton of WEEE recycled avoids the emission of about two tons of CO2.
Let’s ask ourselves now if it is really necessary to always have the latest smartphone model!
Credits:
Author: Maria Beatrice Lupi. A naturalist and expert in training, planning for sustainable development, participatory methodologies, and European planning. Currently, she is involved in dissemination and education for sustainability.
Translation by Maria Antonietta Sessa


